By Rupin Chopra and Shantam Sharma
Introduction:
India, with its vibrant population, welcomes approximately 25 million new lives each year, accounting for about 20% of global childbirths[1]. Nutrition plays a pivotal role in the healthy growth and development of these children. Recently, the Food Safety and Standards Authority of India (FSSAI) has initiated a comprehensive investigation following concerns raised by the Union Consumer Affairs Ministry.
The probe addresses reports alleging that prominent international brands have been marketing sugar-laden infant food products in India, which purportedly fall short of global nutritional benchmarks1. This scrutiny aligns with FSSAI’s commitment to upholding food safety and standards, ensuring that infant nutrition products meet stringent safety and quality standards.
Why did the FSSAI order probe against top infant food manufacturers?
The FSSAI has broadened its scope of monitoring to encompass all brands that sell baby food and infant formula milk in light of the recent allegations[2] against leading brands for impropriety and false claims in baby food products, specifically allegations made by the Swiss investigative group Public Eye and the International Baby Food Action Network that some brands adds sugar to its infant formula in India.
The scientific committee of the FSSAI intends to assess compliance of all such manufacturers, and is presently investigating the issue.
Regulating Laws & Regulations for Infant Food and Nutrition Levels:
As the FSSAI delves into the allegations against major brands, the focus sharpens on the following regulatory framework that governs infant nutrition:
- Infant Foods, Feeding Bottles, and Milk Substitutes (Regulation of Production, Supply and Distribution) Act[3] : India adopted the Infant Foods, Feeding Bottles, and Milk Substitutes (IMS) Act in 1992. In order to tighten up on several provisions and plug any gaps that infant formula manufacturers may have discovered, it was further modified in 2003. All forms of food promotion targeted at children under two years old are strictly prohibited by the IMS Act. Additionally, these infant formula businesses are prohibited from sponsoring medical practitioners and organizations.
- Food Safety & Standards (Packaging and Labelling) Regulations, 2011[4]:
- In compliance with the Food Safety & Standards (Packaging and Labelling) Regulations, 2011, foods intended for infant nutrition must be packaged in hermetically sealed, hygienic, and sound containers or in a flexible pack composed of paper, polymer, or metallic film.
- The infant formula category for any special medical purposes allowed under these regulations must be exempted from the provisions specified under the Regulation of the Food Safety & Standards (Packaging & Labelling) Regulations, 2011. It must be packed with inert chemicals to prevent the contents from deteriorating. Additionally, the label must include a warning if any components that are known to cause allergies are present.
- Food Safety & Standards (Food Products Standards & Food Additives) Regulations, 2011[5]:
- The infant formula category food for infant based on traditional food ingredients provided under these regulations shall conform to the microbiological standards for milk & milk products of category infant formula as given under Appendix B of the Food Safety & Standards (Food Products Standards & Food Additives) Regulations, 2011.
- Source compounds for vitamins, minerals, and other nutrients from Schedule I (a), Schedule I (b), and Schedule I (c), as permitted by regulations, shall be used in the food intended for baby nutrition.
- Algal and fungal oil as a source of Arachidonic Acid (ARA) and Docosahexaenoic Acid (DHA) from Mortierella alpina, Crypthecodinium cohnii, Schizochytrium sp., and Ulkenia sp. at a maximum of 0.5% must be present in the food intended for baby nourishment. The DHA of total fatty acids and the minimal 1:1 ratio of DHA to ARA. In the event that a claim concerning the addition of DHA is made, the DHA level must be less than 0.2% of total fatty acids. Premature babies’ replacements for breast milk cannot include less than 0.2%. DHA of total fatty acids and a minimum 1:1 ratio of DHA to ARA.
- Legal Metrology (Packaged Commodities) Rules, 2011[5]:
All requirements of the Legal Metrology (Packaged Commodities) Rules, 2011 must be met by food intended for infant nourishment, with the exception of the requirement for a standard pack size in line with the second schedule when it comes to infant formula used for any unique medical purpose. - Food Safety and Standards (Food for Infant Nutrition) Regulations, 2020[6]: The FSSAI in 2020 introduced these regulations. These regulations relate to the health and nutrition of infants under the age of twelve months. While they were formerly a part of the Food Safety & Standards (Food Products Standards & Food Additives) Regulations, 2011, the FSSAI subsequently created and implemented a distinct regulation.
Salient Features for Infant Food Regulations under the Food Safety and Standard (Food for Infant Nutrition) Regulations, 2020:
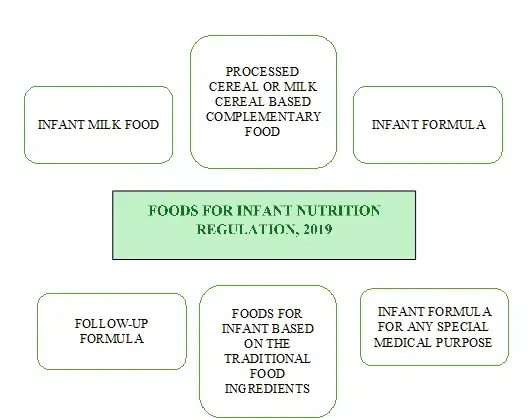
These regulations specify requirements for infant formula intended for specific medical purposes, particularly meals for young children who are born with inborn metabolic errors (IEMs). These include lactose and sucrose-free infant milk replacements that are prepared under infant formula for specific medical needs. The standards for food for infant nutrition based on traditional food sources have also been mentioned in these regulations.
Conclusion:
In light of increased scrutiny by both the FSSAI and foreign agencies, it is crucial for Indian as well as foreign brands operating in India to ensure compliance with the aforementioned laws and regulations. Adherence and effective enforcement of these laws and regulations will ensure that trust is build among consumers and the products that are purchased are safe & of the highest quality.
In the end, it is not merely about compliance but about the health and future of the youngest members of our society:
Aishwarya Rajput, Intern at S.S. Rana & Co. has assisted in the research of this article.
[1] Available at https://www.unicef.org/india/key-data
[2] Available at https://www.livemint.com/companies/news/fssai-to-inspect-samples-of-spices-and-infant-food-panindia-11713793589477.html
[3] Available at https://www.indiacode.nic.in/handle/123456789/1958?view_type=browse
[4]Available at https://www.fssai.gov.in/upload/uploadfiles/files/Packaging_Labelling_Regulations.pdf
[6] Available at https://www.fssai.gov.in/upload/uploadfiles/files/Compendium_Food_Infant_04_10_2022.pdf
[7] Available at https://www.fssai.gov.in/upload/uploadfiles/files/Compendium_Food_Additives_Regulations_08_09_2020-compressed.pdf


