By: Isheta Srivastava & Harshita Mall
Introduction
Disease outbreaks always have devastating effect on humanity. Coronavirus pandemic aka Covid 19 is the one we are going through. Currently, the worldwide confirmed cases of coronavirus infection count for more than a million cases and over a hundred thousand deaths reported to WHO[1].
The novel Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) also known as Coronavirus Disease-19 (COVID-19), belongs to the family of Coronaviridae, the largest group of enveloped viruses causing respiratory and gastrointestinal infections. The biological genetic material of these enveloped viruses posses a single-stranded, positive-sense RNA viruses. RNA of the viruses can be directly translated into proteins in the host cells. Bats are considered as the natural host for Coronaviruse[2]. Apart from SARS-CoV-2, other viruses related to betacoronaviruses family that are known for animal to human transfer are Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV).
COVID-19 was first identified in Wuhan, a capital city of Hubei province in China. The outbreak was declared a Public Health Emergency of International concern (PHEIC) on January 30, 2020 and later a world pandemic by WHO on March 11, 2020. Till today, no drugs or vaccines have been approved by FDA for treatment of COVID-19 infection. The pharmaceutical companies across the globe are working together to develop therapeutic drugs and vaccines. Currently, there are approximately 70 vaccine candidates in pre-clinical trials as per the data provided through a landscape draft by WHO[3].
Patents trends related to therapeutic drugs and vaccines during a disease outbreak
Earlier outbreak of SARS-CoV and MERS-CoV identified in year 2003 and 2012 respectively provided the world with several anti-viral drugs and successful vaccines to cope with virus of same betacoronavirus family. Currently to combat the Coronavirus pandemic, the earlier technologies and existing patents on vaccines are being reviewed and screened to develop therapeutic vaccines for novel SARS-CoV-2. As per the record, there are approximately 120 relevant patents on Coronavirus vaccines that have been published worldwide during the year 2014-2019. The trend of filing shows a rise in number of patents filed during disease outbreaks of SARS and MERS viruses (Fig. 1). (Please note that the patent analysis shown via graph below is done using the Espacenet, a patent search database).
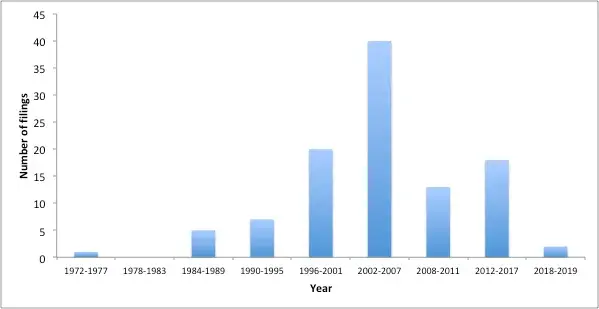
Figure 1 Histogram showing the year wise Patent Filing (Source: https://worldwide.espacenet.com)
Further, legal status of patents filed on human Coronavirus vaccines can be used to infer the status of granted patents that are active or dead. Pie-chart shown in figure 2 illustrates the number of patents and their current legal status. Currently, about 51 percent of the filed patents are granted and active. However, 13 percent of the patents are still pending to be granted. Remaining 36 percent of the patents are either expired, lapsed or revoked (Fig. 2). (Please note that the patent analysis shown via pie chart below is done using the Espacenet, a patent search database)
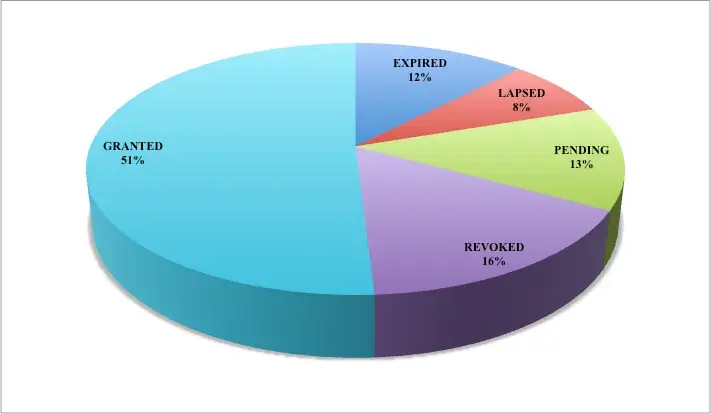
Figure 2 Pie-chart showing the legal status of patents analysis on the patents filed (Source: https://worldwide.espacenet.com)
A closer analysis on the spatial distribution of patents filed worldwide (as per the search data extracted from espacenet) indicates that the top three patent filing countries in this domain are Europe (76), United States (75), and China (47) respectively. In view of the first reported case of SARS-CoV-2 (December 2019) in Wuhan, Hubei province of China, it has six patents specifically filed on the vaccines for human SARS coronavirus (Table 1). ((Please note that the patent analysis shown in the table below is done using the Espacenet, a patent search database).
Table 1 List of Patents on coronavirus vaccines filed by various institutes of Wuhan, China (Source: Espacenet)
| S.No. | Title | Inventors | Applicants | Publication Number | Earliest Priority Date |
| 1. | Gene vaccine for anti SARS coronal virus and use thereof | Ding Hong Ye Kai |
Univ Wuhan | CN1449826A | 2003-05-27 |
| 2. | SARS-Cov gene vaccine based on epi-position and its contruction | Wang Xiaohua Wang Ying Xiong Sidong Sidong Xiong Xiaohua Wang Ying Wang Yiwei Chu |
Univ Fudan Univ Fudan |
CN100588430C | 2004-02-20 |
| 3 | DNA vaccine pVPH for SARS virus | Guo Yingjun Huang Li Sun Shuhan |
No 2 Military Medical Univ Of China | CN1449827A | 2003-06-03 |
| 4 | Immunological adjuvant, and its application in preparing vaccine and medicine for anti-virus | Tian Bo Gao Bo Tian Fu Gao Hongtao Li Minghai Zhou Yuxia Zhang |
Chinese Acad Inst Microbiology | CN100467063C | 2004-07-07 |
| 5 | Heat shock protein 65-human SARS coronary virus epitope antigen recombinant fusion protein (HSP65SARS/3CL161-264) | Wang Liying Tian | Diwei Huayu Biolog Technology | CN1749277A | 2004-09-14
|
| 6 | Novel specific polypeptide and application thereof in preparing medicament for diagnosing, preventing and treating severe acute respiratory syndrome caused by coronaviruses | Dong Huang [Cn] Weiguo Jia [Cn] Huang Dong Jia Weiguo William Campbell Zhao Shumin |
Beijing Genovax Biotechnology Yu Li Zhao Shumin |
CN101575361A | 2009-03-20 |
Pharmaceutical companies and current Coronavirus Pandemic
During the recent pandemic crisis, several global pharmaceutical companies are working in collaboration with research institutes for the development of vaccines to fight the COVID-19 outbreak. At present, there is no anti-SARS-CoV-2 drug to treat the infected population. Some medical practitioners have come up with the strategy to use old anti-viral and anti-malarial drugs for therapeutic purpose. Drugs such as, Remdesivir (Geliad Sciences) and hydroxychloroquine (FDA approved) are being used for the treatment of infected patients. These drugs have been patented for their inhibitory effect on Malaria, SARS-CoV and MERS-CoV and Ebola virus infection. In light of current situation, WHO have published a detailed landscape on the drug candidates and therapeutic products that are being used for treatment of COVID-19 patients and are under clinical trials.
Amidst the crisis, 21 pharmaceutical companies globally have initiated the development of 70 Coronavirus vaccines and therapeutics to combat COVID-19 infections. The major players in the global vaccines market are Merck & Co., Inc., Sanofi, Pfizer Inc., GlaxoSmithKline PLC, and Johnson & Johnson and they secured about 76% revenue share in the global vaccines market. Presently, two US based Geliad Sciences Inc. and Moderna Inc. along with other small biotech companies including three Indian companies Zydus Cadila, Serum Institute and Bharat Biotech are working on developing therapeutics and vaccines for novel Coronavirus. Moderna Inc. a pioneer biotechnology company is in Phase-1 clinical trial to create a new generation of transformative mRNA based vaccine (mRNA-1273) against novel coronavirus (SARS-CoV-2). Further along in vaccine development are CanSino Biologics Inc. of Hong Kong and Beijing Institute of Biotechnology, which are in Phase-1 of clinical trials. The drug makers are working at remarkable speed and started human trials of its vaccines (Table 2). According to a recent report, a team of Italian company Advent-IRBM and Jenner Institute of the University of Oxford has accelerated human testing of vaccine for Phase-1 of clinical trials. If the clinical trials are successful, the vaccine is expected to be ready for use as early as September, 2020.
Table 2 Vaccines under Phase-1 stage of clinical evaluation
| Platform | Types of candidate vaccine | Developer | Corona-virus target | Current stage of clinical evaluation/regulatory status- Coronavirus candidate | Same platform for non-Coronavirus candidates |
| Non-Replicating Viral Vector | Adenovirus Type 5 Vector | CanSino Biological Inc. and Beijing Institute of Biotechnology | COVID-19 | Phase 1
ChiCTR2000030906 |
Ebola |
| RNA | LNP-encapsulated mRNA | Moderna/NIAID | COVID-19 | Phase 1
NCT04283461 |
Multiple candidates
|
The Pharmaceutical companies have worked together with the intellectual property pools in the past (e.g. HIV/AIDS) to ensure that lifesaving treatments and vaccines are available in world market to fight various disease and endemic situations. In this pandemic crisis, the World Health Organization jointly with Unitaid, a UN-backed group funding global health innovation, have proposed the major pharmaceutical companies to share their intellectual property for all medical treatment, vaccines and diagnostics to fight novel coronavirus.
In the post COVID scenario, Indian Pharmaceutical sector is going to play a significant role in fulfilling the global demands of vaccines and drugs as it has current pharmaceutical export of $22billions in FY-20199.
India is among the top 12 countries in global biotechnology industry and with coronavirus pandemic outbreak initiated in China, the global demand for manufacturing vaccines and drugs is expected to surge in coming time. Thus, there is a golden opportunity for India to emerge as major drug manufacturing country when the entire world is likely to shift manufacturing units from China to India.
References:
- Yang, Penghui, and Xiliang Wang. “COVID-19: a new challenge for human beings.” Cellular & Molecular Immunology (2020): 1-3.
- Denise M Hinton (28 March 2020). “Request for Emergency Use Authorization For Use of Chloroquine Phosphate or Hydroxychloroquine Sulfate Supplied From the Strategic National Stockpile for Treatment of 2019 Coronavirus Disease”. US Food and Drug Administration. Retrieved 30 March 2020.
- .Shi Zhengli; Team of 10 researchers at the WIV (4 February 2020). “Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro”. Cell Research. 30 (3): 269–271. doi:1038/s41422-020-0282-0. PMC7054408. PMID 32020029
- https://www.marketwatch.com/story/these-nine-companies-are-working-on-coronavirus-treatments-or-vaccines-heres-where-things-stand-2020-03-06
- https://www.visiongain.com/report/top-20-vaccines-manufacturers-2019/
- https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-first participant-dosed-nih-led-phase-1-study
- https://www.who.int/blueprint/prioritydiseases/keaction/Table_of_therapeutics_Appendix_17022020.pdf?ua=1
- https://www.aa.com.tr/en/europe/italy-uk-team-hopes-to-have-covid-19-vaccine-in-sept/1804516
- https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/indian-pharma-exports-may-touch-22-billion-in-fy-20-pharmexcil/articleshow/71111944.cms?from=mdr
[1] https://www.who.int/emergencies/diseases/novel-coronavirus-2019
[2] https://www.ncbi.nlm.nih.gov/pubmed/32015508
[3] https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape-ncov.pdf?ua=1