Mattel Copyright Infringement Case- India.

Hon’ble Mrs. Justice Mukta Gupta, of the Delhi High Court passed an order dated October 13, 2020 in CS (COMM) NO. 447 of 2020 titled as ‘Mattel Inc. v. Present Enterprises and Ors’ and directed the Defendant No.4, Flipkart to remove the listings which relate to the advertisement and sale of the products which violate the copyright of the Plaintiff i.e. Mattel in the six characters of the ‘Rainforest Family’.
Counterfeiting of Anti-Covid Medicines.
 With India currently engulfed in an all-encompassing firestorm that is the second wave of the COVID-19 pandemic, a corresponding new wave of counterfeiters have emerged side by side, who are trying to benefit from the desperate demand for COVID medicines, by selling counterfeits of life-saving drugs, including the critical Remdesivir.
With India currently engulfed in an all-encompassing firestorm that is the second wave of the COVID-19 pandemic, a corresponding new wave of counterfeiters have emerged side by side, who are trying to benefit from the desperate demand for COVID medicines, by selling counterfeits of life-saving drugs, including the critical Remdesivir.
VAC-SINNERS @ WORK: THE RISE OF ONLINE VACCINATION SCAMS.
 With the COVID-19 pandemic at its peak and subsequent waves still ravaging parts of the world, the need for vaccines, infrastructure and information to combat the virus has escalated manifold. Governments and pharmaceutical companies are now working hand-in-hand to ramp up production and distribution of vaccines to prevent further contagion amongst the population.
With the COVID-19 pandemic at its peak and subsequent waves still ravaging parts of the world, the need for vaccines, infrastructure and information to combat the virus has escalated manifold. Governments and pharmaceutical companies are now working hand-in-hand to ramp up production and distribution of vaccines to prevent further contagion amongst the population.
US puts India on Priority Watch list- USTR Special 301 Report.
 Taking suo moto cognizance of the alarming rate in which Covid-19 cases have increased in India, the Full Bench of the Hon’ble Delhi High Court in Court on its own motion vs. State (Govt. of N.C.T. of Delhi)proceeded to extend the validity .
Taking suo moto cognizance of the alarming rate in which Covid-19 cases have increased in India, the Full Bench of the Hon’ble Delhi High Court in Court on its own motion vs. State (Govt. of N.C.T. of Delhi)proceeded to extend the validity .
STRIKING OF DEFENSE IN COMMERCIAL SUITS.
 The Office of the United States Trade Representative (USTR) on April 30, 2021 has released its Special 301 Report of 2021. The USTR annually releases the Report highlighting the various adequacies, effectiveness as well as shortcomings in the Intellectual Property protection and enforcement regime of various countries which are the Trading partners of the US.
The Office of the United States Trade Representative (USTR) on April 30, 2021 has released its Special 301 Report of 2021. The USTR annually releases the Report highlighting the various adequacies, effectiveness as well as shortcomings in the Intellectual Property protection and enforcement regime of various countries which are the Trading partners of the US.
Mattel Copyright Infringement Case- India.

By Lucy Rana and Priya Adlakha
Hon’ble Mrs. Justice Mukta Gupta, of the Delhi High Court passed an order dated October 13, 2020 in CS (COMM) NO. 447 of 2020 titled as ‘Mattel Inc. v. Present Enterprises and Ors’ and directed the Defendant No.4, Flipkart to remove the listings which relate to the advertisement and sale of the products which violate the copyright of the Plaintiff i.e. Mattel in the six characters of the ‘Rainforest Family’.
Mattel Inc. v. Present Enterprises and Ors
Mattel (hereinafter referred to as the Plaintiff) is in the business of selling games, toys etc. for children since the year 1945. In the year 1993, Plaintiff merged with Fisher-Price and since then is one of the biggest manufacturer and seller of the children/toddler’s toys world-over. The Plaintiff claims to have adopted the trademark ‘KICK AND PLAY’ for game and playthings in the year 2010 and started using the said trademark in India since 2012. In the year 2012 the Plaintiff also designed and adopted a set of cartoon animal characters titled as ‘Rainforest Family’ which consists of Rainforest Elephant, Rainforest Lion, Rainforest Giraffe, Rainforest Girl Monkey, Rainforest Parrot & Rainforest Turtle. The same are illustrated below:

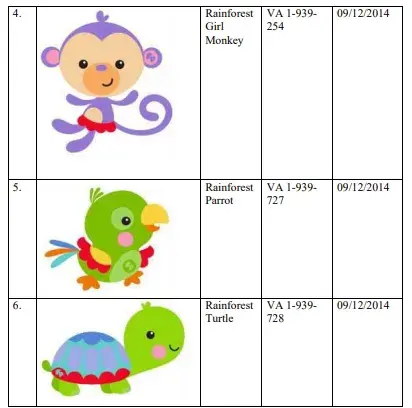
As per the Plaintiff, the Plaintiff in the year 2017, launched the ‘Infant to Toddler Rocker’ with ‘Rainforest Family’ characters featuring therein which products were launched in India in 2018. Further, the Plaintiff applied for registration of its trademark ‘KICK AND PLAY’ in Class-28 in India on August 13, 2020 and applied for the registration of its shape mark of its ‘KICK AND PLAY’ baby gym in Class-28 in India on September 9, 2020.
In the present suit, the Plaintiff claims infringement of its copyright in respect of its six characters of ‘Rainforest Family’, as also passing off the goods of the Defendants as that of the Plaintiff’s by using trademark ‘KICK AND PLAY’ as also the violation of the shape mark of its ‘KICK AND PLAY’ baby gym.
Submissions
The Plaintiff argued on the provisions of Section 40 of the Copyright Act, 1957 that deals with the power to extend copyright to foreign works. Plaintiff submitted that the series of the six cartoon animal characters were granted copyright in the jurisdiction of the United States of America and by way of the official order dated March 24, 1999, would also extend to the Indian jurisdiction in respect of International Copyright.
Observations
The Court observed that a bare perusal of the six characters shows that they have been uniquely prepared with the unique colour combination which is attractive and appealing to the children and by virtue of Section 40 of the Copyright Act, registrations in favour of the Plaintiff in respect of six characters in USA would also extent to India in terms of International Copyright order notified in the Official Gazette on March 24, 1999. In view thereof, the Hon’ble Court was pleased to pass the following order in favour of the Plaintiff:
- Ex-parte interim injunction granted by the Delhi High Court with respect to Copyright –The Hon’ble Court granted an ex-parte ad-interim injunction in favour of the Plaintiffs and against the Defendants. Nos. 1, 2, 3 and other Defendants sought to be impleaded as John Doe as Defendant No.5, against violating of the Plaintiff’s Copyright in six animal characters of the ‘Rainforest Family’ in any manner till the next date of hearing. It may be noted that the Hon’ble Court was of the view that any ad-interim injunction with respect to trademark violation of Plaintiff’s word mark ‘KICK AND PLAY’ as also the design mark, to be given only after the Defendants have been granted an opportunity of hearing.
- Flipkart (Defendant No.4) ordered to remove listings of the products infringed – In addition to the injunction ordered, the Court also directed Defendant No. 4, namely the leading e-commerce platform, Flipkart to remove listing from its platform which relate to the advertisement and sale of the products which violate the copyright of the Plaintiff in the six characters of the ‘Rainforest Family’ within 48 hours from the date of the said order.
At present, Flipkart, i.e. after complying with the order dated October 13, 2020 in the present matter, preferred an application under Order 10 Rule 1 seeking deletion of its name from array of parties as it has complied with the direction of the Court. The said application is still pending adjudication before the Hon’ble Delhi High Court.
Related Posts
TRADEMARK INFRINGEMENT- NIKE v. MSCHF- SATAN SHOES CASE
Internet Service Provider’s Liability for Copyright infringement in India Law
Counterfeiting of Anti-Covid Medicines.

By Ragini Ghosh and Pranit Biswas
With India currently engulfed in an all-encompassing firestorm that is the second wave of the COVID-19 pandemic, a corresponding new wave of counterfeiters have emerged side by side, who are trying to benefit from the desperate demand for COVID medicines, by selling counterfeits of life-saving drugs, including the critical Remdesivir.
While the nation grapples with this unprecedented human tragedy and loss of life, the last few weeks have seen a rise in counterfeiting of such drugs, as evidenced by the many recent instances of law enforcement nabbing such counterfeiters. Recently on April 17, the Bengaluru police arrested a counterfeiter who was purportedly trying to sell counterfeit Remdesivir in the black market for an exorbitant sum of Rs. 14,000/- per vial. There have been a spate of such incidents which have been reported in the Indian media. It is heart wrenching to see that counterfeiters are not even sparing those in desperate, life-threatening condition during this human catastrophe to reap illegal profits. Similar incidents have also been reported in Pune, Indore, Mumbai, etc., thereby showing the alarming degree of proliferation of such unscrupulous activities. In addition to acts of counterfeiting, there have also been reports of crude attempts to even try and pass-off saline water as Remdesivir!
It is therefore becoming increasingly imperative at this time that the general public be made aware of basic due diligence measures that can be conducted to try to spot fake medicines, especially critical care drugs such as Remdesivir at a time when there appears to be NO HONOUR amongst thieves, (unlike what the title of Mr. Archer’s namesake novel would have us believe!)
FAKE vs. REAL- Remdesivir

Source- https://mobile.twitter.com/manabhardwaj/status/1386578612707643393
Remdesivir in India is manufactured by several pharma companies, one of the principal of which is Hetero Healthcare Limited, a Hyderabad based pharmaceutical manufacturer, marketing the drug under the brand name COVIFOR. A senior officer of the Delhi Police recently released comparative photographs and tips about how to spot fake COVIFOR from original over Twitter. The same is being shared below for increasing the visibility of such information, which may turn out to be the difference between life and death, when battling COVID-19.
How to spot fake COVIFOR from original?
| ORIGINAL | FAKE/COUNTERFEIT |
| · The term “Rx” is mentioned on the top left, as a superscript, above the name Remdesivir.
|
· The counterfeit package does not have the term “Rx”. |
| · In the word “Vial”, the “V” is in uppercase script/capitalised. | · The alphabet “V” in the word “vial” on the counterfeit packaging is not capitalised. |
| · The mark COVIFOR is placed horizontally in the center, but a bit higher up from the vertical-middle.
|
· The mark COVIFOR herein appears to be printed with a different alignment. |
| · The statement in the bottom of the front side of the packaging, reads as “For use in Hospital/ Institutional set up only”. Herein the first alphabet, i.e. ‘F’, is capitalized.
|
· The alphabet “F” is not capitalized in the fine print statement at the bottom of the front face of the counterfeit packaging. |
| · The original packaging has the instruction “FOR INTRAVENOUS INFUSION ONLY“.
|
· The counterfeit packaging does not seem to advertise this instruction. |
| · The description ‘Lyophilized Powder’ is mentioned above the mark COVIFOR.
|
· This is not mentioned on the counterfeit packaging. |
| · The sentence ‘Powder for concentrate for solution for infusion‘ is printed below the mark COVIFOR. | · This statement is not there below the COVIFOR mark on the counterfeit packaging.
|
| · The original has a warning label on the back of the box, which is in red colour.
|
· The warning on the counterfeit packaging is in black coloured font. |
| · COVIFOR/ Remdesivir was developed made by Gilead Sciences (an American Company), and a label stating “Covifor is manufactured under a licence from Gilead Sciences, Inc.” is printed at the back of the original packaging, below the abovementioned red coloured warning. | · No such statement is printed on the back- of the counterfeit packaging.
· The backside has another capitalization error – under the Indian manufacturer’s name, ‘India’ is written as ‘india’, i.e. without the alphabet ‘I’ being in uppercase. · The state ‘Telangana’ is misspelt as ‘Telagana’.
|
It is hoped that the above non-exhaustive list of pointers will help the general public to exercise vigilance and be able to distinguish counterfeit pharmaceutical products from real ones. In addition, consumers must also be mindful of the below factors:
- Difference in MRP (as widely reported) for such drugs, compared to the price being offered to them – whether on the higher side or lower side;
- Quality of packaging – it may be possible that counterfeiting packaging may be of poorer quality, the shape, print-colours may be slightly different, etc.
- Check spellings of all discernible text – it is unlikely that the original packaging/vials would have typographical errors (for example, Telangana misspelt as Telagana).
Considering the scale and magnitude of this second wave of COVID-19, it is also important to be equally vigilant while buying critical PPE (Personal Protection Equipment) such as gloves or masks. In this regard, you may access our earlier articles on the said topic at https://ssrana.in/articles/proliferation-of-counterfeits-during-covid-19-lex-witness/ and https://ssrana.in/articles/rise-of-counterfeit-ppe-india-covid-19/.
The country, as a whole, must be made aware and exercise caution and vigilance against such counterfeiting activities in the midst of an unprecedented crisis as the present one that we are facing. Besides the law enforcement authorities cracking down against such counterfeiters, it is also the prerogative of brand owners and IP holders (such as vaccine makers, manufacturers, etc.) to take pro-active action against such counterfeiters, which not only would save countless lives, but also safeguard their own valuable reputation and intellectual property.
Related Posts
Counterfeiting in Indian Industries
Intellectual Property Laws and Counterfeiting in India
VAC-SINNERS @ WORK: THE RISE OF ONLINE VACCINATION SCAMS.

By Lucy Rana and Sanjana Kala
With the COVID-19 pandemic at its peak and subsequent waves still ravaging parts of the world, the need for vaccines, infrastructure and information to combat the virus has escalated manifold. Governments and pharmaceutical companies are now working hand-in-hand to ramp up production and distribution of vaccines to prevent further contagion amongst the population. However, it wasn’t long before health and state bodies had to face the plague of misinformation spread by nefarious elements. This accompanied with the immense amount of panic amongst people has now fuelled a trend of counterfeits and opportunistic fraud committed by people looking to take advantage of the gap in supply and demand. Interestingly, the pandemic has seen a rise in instances of fraud and counterfeiting perpetuated, in large part, by online channels such as:
- Fraudulent domain name registrations or cybersquatting;
- Hoax websites mirroring the look and feel of genuine companies engaged in healthcare;
- Fake listings on the dark web;
- Misleading messages on social messaging applications;
- Search algorithms and paid advertisements on online search engines;
- Fake listings on social media platforms;
- Phishing emails impersonating government authorities;
- Fake requests for charitable donations;
This article focuses on the rise of fraudulent domain name registrations and illegal websites that have cropped up specifically after the announcement of largescale vaccination drives all around the world.
FRAUDULENT DOMAIN NAME REGISTRATIONS
WIPO in a recent decision in Pfizer Inc. v. Registration Private, Domains By Proxy, LLC / Juan Beltran[1] dated March 18, 2021 relating to the domain <pfizermx.com> found opportunistic bad faith on part of the Respondent owing to the fact that the impugned website (linked to the aforesaid domain name) mirrors Pfizer’s website “www.pfizer.com” and falsely purports to offer Pfizer’s COVID-19 vaccine for sale. The domain <pfizermx.com> was thereafter transferred to the Complainant, Pfizer.
It is pertinent to mention that in 2020 WIPO handled 4,204 cybersquatting cases which was nearly a 14% increase over the year 2019.
Cyber squatters are now not only using similar/identical domain names but are also using another technique called ‘typosquatting’ wherein a very minute and purposeful change is made to the original domain name by changing the font and/ or spelling of a single letter in the domain so as to make the said change undetectable to an average consumer.
DomainTools reported that more than 150,000 new, high risk COVID-19-themed domains have been registered since December 2019. The report stated that since the most valuable space in the internet is .com, it is also the most valuable space to carry out typosquatting. Further, it was revealed that the industry’s most attractive domains for typosquatters to target are financial institutions or organizations that sell medicine.[2] As per Cybercrime Magazine’s data feed file for February 17, 2020, seven more newly registered domains with the keyword “coronavirus” were revealed as below:
- coronavirus-insurance.com
- com
- world
- earth
- com
- com
- com
ILLEGAL WEBSITES
The National Intellectual Property Rights Coordination Center, (an investigative arm of the U.S. Department of Homeland Security) has removed 30 websites and seized 74 web domains in connection with fraud related to the Covid-19 pandemic.[3] As per an investigation by the U.S. Department of Homeland Security, a sham website was offering doses of Moderna’s Covid-19 vaccine for $30 each and claimed that consumers may be able to buy a Covid-19 vaccine ahead of time. Other fake websites posing as Pfizer Inc. and BioNTech SE have also popped up prompting U.S. Homeland Security to analyse almost 80,000 Covid-19 domain names as of February, 2021.[4]
The United States, Mexico and other countries have seized and taken down dozens of websites fraudulently claiming to sell shots or an affiliation with vaccine makers such as Moderna and Pfizer. The fake, company look-alike websites appeared to be seeking consumers’ personal information to be used in identity-fraud schemes.[5] These scam attempts also involved simple tools and everyday payment methods, often luring unsuspecting consumers through paid advertisements and search algorithms on search engines such as Google. One such instance is the fraud website at www.coronavirusmedicalkit.com which claimed to sell vaccines for COVID-19 by way of DIY vaccines kits containing a non-existent vaccine and instructing consumers to administer it with water. The website carried photographs of the purported medical kit along with a Fedex form asking for the customers’ credit card information. The website was then shut down by the U.S. Department of Justice[6].
Another growing trend is mimicking companies manufacturing leading COVID-19 vaccines with the aim to collect personal information of consumers to carry out phishing attacks. For instance, a fake website under the name www.regeneronmedicals.com claimed to be linked to Regeneron Pharmaceutics Inc., (the biotechnology company that provided the vaccine used on former President Trump). Another fake website at www.mordernatx.com had the look and feel of Moderna Inc’s actual website, www.modernatx.com.[7]
LEADING VACCINES AND POSSIBLE ONGOING FRAUDS
CovaXin is primarily developed by Bharat Biotech & ICMR and is widely used in countries such as India, Nepal, Iran, Mauritius, Mexico etc. Information about this vaccine is available at https://www.bharatbiotech.com/covaxin.html. However, possible unauthentic domains appear to have cropped up in this regard:
- <in>: A suspicious .IN TLD under the name <CovaXin.in> appears to be registered by an entity in Bihar. No content as of yet is being hosted therein.
- < com>: This domain, registered in March 2020 by an entity in Karachi, resolves to a parked page.
Covishield manufactured by the Serum Institute of India and jointly developed by Oxford University and AstraZeneca is the cheapest vaccine in the world. Further information about this vaccine is available at https://www.seruminstitute.com/product_covishield.php. A possible fraudulent domain name registration in this regard appears to be available at:
- <co.in>: Registered in July 2020 by a third party in India, no content appears to be hosted on the website for this domain as of yet.
CoronaVac, also known as the Sinovac COVID-19 vaccine, is a vaccine developed by the Chinese company Sinovac Biotech. Some possible fraudulent domain name registrations in this regard have been listed below:
- <com>: This domain was created on September 2020 and currently resolves to a parked page. Notably, this domain is listed for sale.
- <in>– This domain appears to be registered by an entity located in Uttar Pradesh dated December 2020. The domain resolves to a parked page.
WAY FORWARD
Such unauthentic domains/websites indulging in illegal and fraudulent activities are aimed at deceiving the general public in order to derive undue monetary gains. Not only are these cyber squatters thriving on the goodwill of an established brand but at the same time putting the life of consumers at risk by exploiting public fear.
Given this, the need of the hour is for health and state bodies to take some steps to mitigate the menace of fraudulent domains and hoax websites in a swift and efficient manner. Some steps in this regard include:
- Propagation of authentic and verified information by government bodies;
- Active enforcement actions by brand-owners against mala fide domain name registrations including filing take-downs and domain name complaints;
- Focus on quick resolution preferably within 24 hours to avoid dissemination of online misinformation.
Consumers may also take some preventive measures and exercise caution whilst perusing data relating to COVID-19 online, in the manner suggested below:
- Avoid online requests for personal information;
- Look out for spelling and grammatical mistakes
- Double check email addresses and link to prevent downloading malware;
- Report any instances of fraud or suspicious online activity;
- Donate only to reliable sources.
Related Posts
Counterfeiting of Anti-Covid Medicines
COPYCAT COVID PRODUCTS – NEW FACE OF COUNTERFEIT
Proliferation of Counterfeits during COVID – 19
Rise of Counterfeit PPE in India amid COVID-19
[1] Case No. D2021-0094
[3] https://www.albawaba.com/news/fake-pfizer-covid-19-vaccine-doses-found-mexico-poland-1423719
[4] https://www.wsj.com/articles/scammers-are-setting-up-fake-covid-vaccine-websites-11614374257
[7] https://www.wsj.com/articles/scammers-are-setting-up-fake-covid-vaccine-websites-11614374257
US puts India on Priority Watch list- USTR Special 301 Report.

The Office of the United States Trade Representative (USTR) on April 30, 2021 has released its Special 301 Report of 2021[1]. The USTR annually releases the Report highlighting the various adequacies, effectiveness as well as shortcomings in the Intellectual Property protection and enforcement regime of various countries which are the Trading partners of the US.
The USTR while releasing the Report remarked that it has reviewed more than one hundred trading partners and placed thirty-two of them on either the Priority Watch List or Watch List.
India has again been put under the Priority Watch List by the USTR, which implies that India’s IPR law, protection and enforcement strategies will be a subject of particularly intense bilateral engagement during the coming year.
Other countries which have featured in the Priority watch list are Argentina, Chile, China, Indonesia, Russia, Saudi Arabia, Ukraine, and Venezuela.
The entire report in detail can be accessed here.
Take Away from Special 301 Report- India
Counterfeit medicines
The Report speaks at length about India being a source of counterfeit medicines. It remarks that the majority of all counterfeit pharmaceuticals seized at the U.S. border in FY 2020 were shipped from or trans shipped through China, Hong Kong, India, Canada, and the Dominican Republic. It also highlights that a recent study by OECD and EUIPO found that China, India, the Philippines, Vietnam, Indonesia, and Pakistan were the leading sources of counterfeit medicines distributed globally.
Inadequate IP Laws
India’s IP protection and enforcement has been recognized as inadequate and as one of the world’s most challenging major economies with respect to IP protection and enforcement. Some of drawbacks listed in the report are:
Slow opposition or cancellation proceedings.
- Potential threat of patent revocations, lack of presumption of patent validity, and the narrow patentability criteria under the India Patents Act burden companies across different sectors.
- Patent applicants continue to confront costly and time-consuming pre- and post-grant oppositions, long waiting periods to receive patent approval, and excessive reporting requirements.
- Vagueness in the interpretation of the India Patents Act.
- Restriction on patent-eligible subject matter in Section 3(d) of the India Patents Act and its impact on incentivizing innovation that benefits Indian patients.
- Weak enforcement of IP by the courts and police officers, a lack of familiarity with investigation techniques, and the continued absence of any centralized IP enforcement agency, combined with a failure to coordinate actions on both the national and state level, threaten to undercut any progress made.
India a home for markets that facilitate counterfeiting and piracy
India remains home to several markets that facilitate counterfeiting and piracy, as identified in the 2020 Notorious Markets List.
Notorious Markets List 2021 for Counterfeiting
Administrative Inadequacies
U.S. brand owners continue to report excessive delays in trademark opposition proceedings and a lack of quality in examination.
Little clarity concerning whether trademark owners can apply directly for recognition of “well-known” trademark status without having to rely on Indian court decisions.
Trade secret protection
The USTR also mentions about India’s lack of laws relating to trade secret protection. It reports that Companies also continue to face uncertainty caused by insufficient legal means to protect trade secrets in India. It remarks that as of 2021 there are no civil or criminal laws in India which specifically addresses the protection of trade secrets protection in India.
Copyright and Piracy
With reference to copyright holders in US, the report states that the Copyright owners report high levels of online piracy.
It also speaks about the draft Copyright Amendment Rules of 2019 which proposes broadening the scope of statutory licensing to encompass not only radio and television broadcasting but also online transmissions. The Report raises the concern that if the Rules are enforced then the Amendment Rules would have severe implications for right holders who make their content available online.
Some positive IP developments reported by USTR
- India’s accession to the World Intellectual Property Organization (WIPO) Internet Treaties in 2018 and the Nice Agreement in 2019.
- India took steps to address stakeholder concerns over burdensome patent reporting requirements by issuing a revised Manual of Patent Office Practice and Procedure in November 2019 and revised Form 27 on patent working in October 2020. The Manual includes the requirement for patent examiners to look to the WIPO Centralized Access to Search and Examination (CASE) system and Digital Access Service (DAS) to find information filed by patent applicants in other jurisdictions, with the aim of eliminating the need for applicants to file redundant information with India.
- In September, 2019, the Nice Agreement for the classification of goods and services for the purposes of registering trademarks came into force, and India continues to work on guidelines for its implementation.
- The Cell for IPR Promotion and Management (CIPAM) continues to promote IP awareness, commercialization, and enforcement throughout India.
- In December 2020, the United States Patent and Trademark Office and DPIIT signed a new Memorandum of Understanding relating to IP technical cooperation mechanisms.
- In March 2021, the United States and India, along with Australia and Japan, announced the Quad Vaccine Partnership. They are taking shared action necessary to expand safe and effective COVID-19 vaccine manufacturing in 2021 and are working together to strengthen and assist countries in the Indo-Pacific with vaccination, in close coordination with the existing relevant multilateral mechanisms including the World Health Organization (WHO) and COVID-19 Vaccines Global Access (COVAX).
- Indian manufacturers have entered into voluntary licensing agreements with international partners to produce billions of COVID-19 vaccine doses. The United States intends to continue to engage with India on IP matters, including through the United States-India Trade Policy Forum’s Intellectual Property Working Group.
Conclusion
India’s IP regime for protection and enforcement of IP rights has always been under the scanner and this is not the first time that India has featured in USTR’s Priority watch list. The past year was a bumpy one for one and all. While health of citizens became paramount concern, India also witnessed some significant changes in the IPR laws favouring the start-ups and innovators in India.
The menace of counterfeiting and piracy acts as a major catalyst in fizzing India’s position with respect to IPR protection. The report also remarks about IPAB’s disbandment and remarks that the United States is closely monitoring legislation proposed in early 2021 that seeks to abolish the IPAB and a temporary ordinance promulgated in April 2021 that effectively disbands the IPAB.


